Which of the Following Statements About Dehydration Synthesis Is False
B Electrons are shared between atoms of the joined monomers. A It has 8 protons in its nucleus.

Solved Which Of The Following Statement About Dehydration Reactions Is False A Water Is Released During The Process B Small Sugar Chains Reply On Dehydration Reactions To Form Larger Polym C Amino Acids
D Covalent bonds are formed between the monomers.

. B It has 8 electrons in its nucleus. C Water is a polar molecule. D Animal digestive systems utilize this process to break down food.
Monomers are joined together by the process of hydrolysis. During a condensation reaction two molecules are condensed and water is lost to form a large molecule. C H 2 O is formed as the monomers are joined.
Question 65 1 1 point Cellulose differs from starch in that the monomers of cellulose are held together by covalent bonds whereas the monomers of starch are held together by hydrogen bonds. Water is formed by dehydration synthesis 2. D Water is formed as a part of a dehydration synthesis reaction.
Which of the following statements about the monomers and polymers found in living organisms is falseThe monomers used to make polymers are essentially universal. Water can break ionic bonds 5. 17 Calculate the number of moles in 92 grams of.
B Water molecules are formed by hydrolysis. B Electrons are shared between atoms of the joined monomers. A H 2 O is formed as the monomers are joined.
Dehydration synthesis is the process of joining two molecules or compounds together following the removal of water. 16 Which of the following statements about the atom 168O is false. A Water is a polar molecule.
A One monomer loses a hydrogen atom and the other loses a hydroxyl group. 15 Which of the following statements about dehydration synthesis is false. C Covalent bonds are formed between the monomers.
134 Which of the following statements about dehydration synthesis is false. E Its atomic weight is 16. D Water freezes from the top down.
C It has 8 neutrons in its nucleus. D Water molecules are formed by hydrolysis. B It has 8 electrons in its nucleus.
B Water is a part of a dehydration synthesis reaction. C One monomer loses a hydrogen atom and the other loses a hydroxyl group. D Covalent bonds are formed between the monomers.
B One monomer loses a hydrogen atom and the other loses a hydroxyl group. A One monomer loses a hydrogen atom and the other loses a hydroxyl group. C Water freezes from the top down.
Which of the following statements is false. C Covalent bonds are formed between the monomers. 3 Which of the following statements is false.
A Water is a polar molecule. 4 Which of the following statements is FALSE. Atomic hydrogen is obtained by passing hydrogen through an electric arc.
A One monomer loses a hydrogen atom and the other loses a hydroxyl group. Hence the untrue statement in the question is that dehydration synthesis results in the breakdown of large molecules into smaller molecules because it is the opposite synthesis of large molecules from small ones. E Salts readily dissolve in water.
This is the same exact process that occurs during a dehydration synthesis. A Salts readily dissolve in water. Trick question since a-d are all false statements.
134 A H 2 O is formed as the monomers are joined. E Water is a polar molecule. Up to 24 cash back 14 Which of the following statements about dehydration synthesis is false.
E Water freezes from the top down. A Salts readily dissolve in water. Finely divided palladium adsorbs large volume of hydrogen gas.
27 Which of the following statements is FALSE. B Water is a part of a dehydration reaction. Requires energy to occur.
Most animals cannot break down cellulose whereas starch is easily digested. E Animal digestive systems utilize this process to break. D Its atomic number is 8.
C Water molecules are formed by hydrolysis. Starch is made of glucose monomers whereas. Pure nascent hydrogen is best obtained by reacting Na with C 2.
Dehydration synthesis builds up large molecules from small ones hence it is an endothermic process ie. B Water is a part of a dehydration synthesis reaction. D Animal digestive systems utilize this process to break down food.
Which of the following statements is false. Cells typically make all of their macromolecules from a set of 40-50 common monomers and a few other ingredients that are rare. Hydrogen gas will not reduce heated aluminium oxide.
14 Which of the following statements about dehydration synthesis is false. Water is a polar molecule 3. Water is a part of a dehydration synthesis reaction water freezes from the top down Water is a polar molecule Salts readily dissolve in water Water molecules are formed by hydrolysis.
Consider the following statements. B Animal digestive systems utilize this process to break down food. Glycogen is formed by plants and cellulose by animals.
B H2O is formed as the monomers are joined. 15 Which of the following statements about dehydration synthesis is false. Water molecules form hydrogen bonds 4.
C H2O is formed as the monomers are joined. Water molecules are formed by hydrolysis.
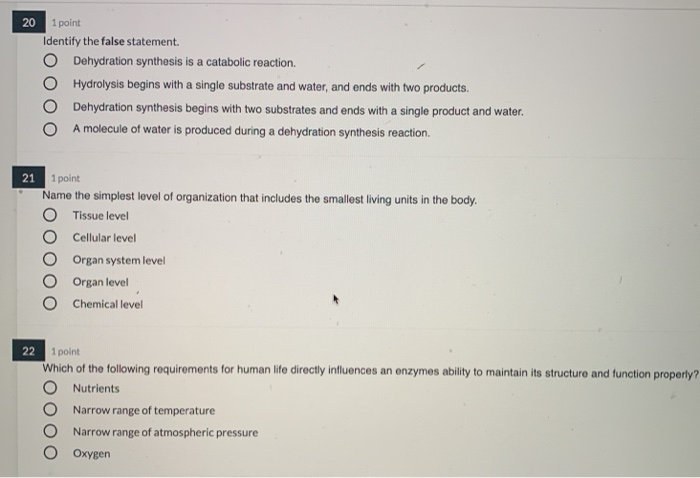
Solved 20 1 Point Identify The False Statement O Chegg Com
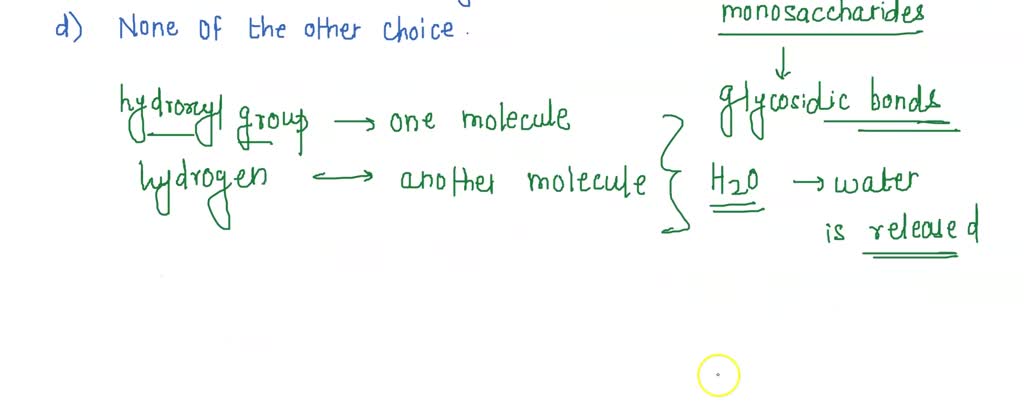
Solved Which Of The Following Statement About Dehydration Reactions Is False A Water Is Released During The Process B Small Sugar Chains Reply On Dehydration Reactions To Form Larger Polym C Amino Acids
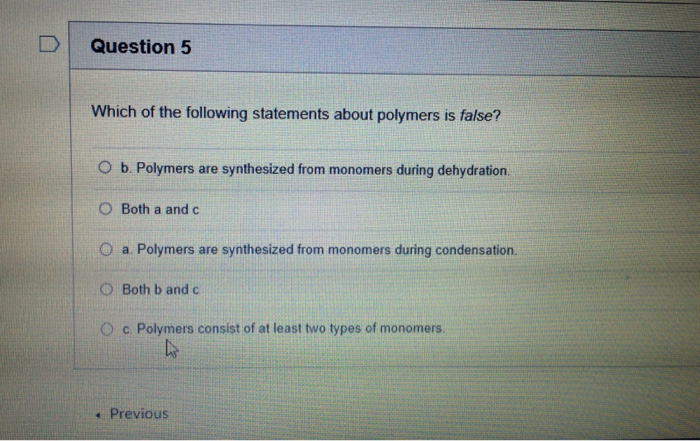
Solved D Question 5 Which Of The Following Statements About Chegg Com
No comments for "Which of the Following Statements About Dehydration Synthesis Is False"
Post a Comment