Which Best Describes the Electrostatic Forces on These Ions
The electrostatic force is an attractive and repulsive force between particles are caused due to their electric charges. See discussion on lattice energy.
Coulomb S Law And Electric Force Review Article Khan Academy
Question 1 Which statement best describes the structure and bonding in SiO2.
. What is similar and different about the formula for gravitation and the formula for electric force. Find the electrostatic force between two such ions when separated by a distance of 4 angstrom in. ℹ DrBob222 May 1 2010 I think I understand but the thing that Im still a bit confused about is how ionic forces and dipoles differ.
Electrostatic force is the force that occurs between electrically charged objects. Chemical Engineering questions and answers. Polarity of Water Molecules.
The Force existing between two charged particles is known as electrostatic force. It is also referred to as Columbs force. The loss of electrons will result in the formation of cation whereas the gain of electrons results in formation of anion.
Question 17 1 1 pts The all-or-none principle associated with the action potential states that. The electrostatic forces have to be overcome to move the ions and shift them away from one another. H 2 F because fluorine is short of two electrons and hydrogen is short of one electron.
The sharing of electrons results in formation of covalent bond whereas transfer of electrons results in the formation of ionic bonds. Ten electrons have been removed from each atom to form ions. An ionic lattice is held together by strong electrostatic forces of attraction between the oppositely charged ions.
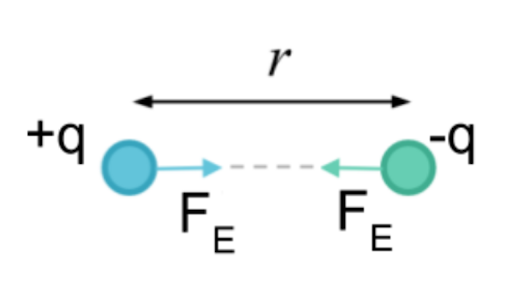
This force is also called the Coulomb force or Coulomb interaction and is so named for French physicist Charles-Augustin de Coulomb who described the force in 17851. The strong electrostatic forces of attraction between oppositely charged ions are called ionic bondsIonic compounds have high melting and boiling points. Its 10 electrons 8 from the oxygen atom and 2 from the two hydrogen atoms tend.
Electric forces can be repulsive. Van der Waals forces. Electrostatic forces are attractive or repulsive forces between particles that are caused by their electric charges.
The electric force between stationary charged bodies is conventionally known as the electrostatic force. Metal atoms form positive ions while non-metal atoms form negative ions. 1 Electrostatic attraction between shared pairs of electrons and positively charged nuclei held together by dipole-dipole forces 2 O Electrostatic attraction between positive cations arrange in a 3D lattice and the surrounding delocalised electrons 3 O Electrostatic attraction.
Electrostatic force binding energy that keeps electrons in their shells as a result of centripetal force and attraction of opposties binding energy electrons determined by distance between nucleus electron in shell negative charge when an atom has more electrons than protons and neutrons positive charge. This is called ionic bonding. As the ionic lattice contains such a large.
What is an Electrostatic Force. If the charge on each of two small charged metal spheres is doubled and the distance between the spheres remains fixed the magnitude of the electric force between the spheres will be. A Iron is composed of macromolecules held together by strong bonds.
Inside the nucleus of an atom there is a competition between two principle forces. Strong nuclear forces which keep the nucleus together and electrostatic forces between the protons which want to. B Iron is composed of atoms held together by delocalized electrons.
Ionic solids are hard and brittle. There is no force on either ion to move. This is due to the presence of other charges in the cell.
The electrical forces between charged particles atomic or molecular ions protons or electrons is one of the four fundamental kinds of forces in the universe the others are gravity strong and weak nuclear forces which we call this electrostatic. Water is a strongly polar molecule. The forces act in all directions in the lattice.
HF because both fluorine and hydrogen are capable of forming only one covalent bond. The two oppositely charged ions are held together by electrostatic force of attraction between them. Ionic compounds are held together by electrostatic forces between the oppositely charged ions.
The sum of the attractive or repulsive forces between molecules or between parts of the same molecule other than those due to covalent bonds or the electrostatic interaction of ions with one another with neutral molecules or with charged molecules. Electrostatic forces are just the attraction for ions to each other as opposed to dipole-dipole ion-dipole London and other types of forces between molecules and clusters of molecules. Was this answer helpful.
Both have constants and are both inversely proportional to the distance of separation squared r2 Differences. These forces are usually referred to as ionic bonding. C Iron is composed of positive and negative ions held together by electrostatic attractions.
HF because both fluorine and hydrogen are capable of forming only one ionic bond. Why electrostatic force is called coulombs force. Because of the strong electrostatic force of attraction among ions in the solid they have high melting points and high boiling points.
Which of the following best describes Fe s. The best example of this charge screening is the water molecule represented as H 2 O. The force on sodium ions is to move out of the cell and the force on potassium ions is to move into the cell.
The reason is that the electrostatic force is diluted due to screening between molecules.

Allred Rochow Electronegativity Chemwiki Teaching Chemistry Chemistry Help Chemistry Lessons

Introduction To Static Electricity Let S Talk Science

Electrostatic Attraction An Overview Sciencedirect Topics

Static Electricity Lab Using Balloons To Show Electrostatic Forces Static Electricity School Science Experiments Middle School Science Experiments
No comments for "Which Best Describes the Electrostatic Forces on These Ions"
Post a Comment